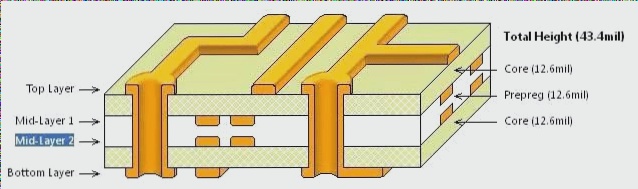
The process of manufacturing printed circuit boards (PCBs) involves a complex series of physical and chemical reactions, culminating in the formation of circuit patterns from the initial light board. This article will focus on the final crucial step: etching.
Currently, the predominant method in PCB fabrication is the “pattern plating method.” This approach begins by applying a layer of lead-tin corrosion-resistant material onto specific areas of the copper foil intended to remain on the outer layer, forming the circuit pattern. Subsequently, the remaining copper foil is chemically dissolved away in a process known as etching.
Types of Etching
It is important to note that during the etching process, the board typically features two layers of copper. In outer layer etching, only one layer of copper is selectively dissolved, leaving behind the required circuit pattern. This pattern plating method ensures that copper plating exists solely beneath the lead-tin resist layer.
Alternatively, another method involves plating copper across the entire board, with areas not covered by photosensitive film treated only with tin or lead-tin resist. Known as the “full board copper plating process,” this method requires copper plating over the entire board surface, followed by complete etching of unwanted copper. Unlike pattern plating, this approach demands two rounds of copper plating across the board, increasing complexity. It becomes particularly problematic with fine wire widths, leading to issues such as side corrosion that can significantly impact line uniformity.

1. In the processing technology of the outer circuit of the printed circuit board, another method involves using photosensitive film as the resist layer instead of metal coating. This method closely resembles the inner layer etching process and can be referenced during inner layer manufacturing.
2. Currently, tin or lead-tin is the most commonly used corrosion-resistant layer in the etching process involving ammonia-based etchants. Ammonia-based etchants are widely used chemical solutions that do not undergo chemical reactions with tin or lead-tin. Ammonia etchants primarily consist of ammonia/ammonium chloride etching solutions.
3. Additionally, ammonia/ammonium sulfate etching chemicals are available in the market. Copper can be separated from sulfate-based etching solutions through electrolysis, allowing for reuse. Due to its low corrosion rate, it is less common in actual production but is anticipated for chlorine-free etching.
4. Some have attempted to use sulfuric acid-hydrogen peroxide as an etchant for corroding outer layer patterns. However, due to reasons such as economic viability and the management of waste liquids, this process has not gained widespread commercial use. Moreover, sulfuric acid-hydrogen peroxide cannot be used for etching lead-tin resist, making it a secondary method in outer layer PCB production, thus attracting minimal attention.
5. Etching Quality and Previous Issues
6. The fundamental requirement for etching quality is the complete removal of all copper layers except those under the resist layer. Strictly speaking, etching quality encompasses wire width consistency and the degree of undercutting. Given the characteristics of current etching solutions, side etching in the horizontal directions is nearly unavoidable.
7. Undercutting is a critical etching parameter often discussed, defined as the ratio of undercut width to etch depth, known as the etching factor. In the printed circuit industry, this factor varies widely, from 1:1 to 1:5. A minimal undercut or low etching factor is generally preferred.
8. Equipment Adjustment and Interaction with Etching Solutions
9. In printed circuit processing, ammonia etching involves delicate and complex chemical reactions. Once optimized, production can proceed smoothly. Continuous operation is crucial; halting and drying are not advisable. The effectiveness of the etching process largely depends on equipment performance.
10. Regardless of the etching solution used, high-pressure spraying is essential for achieving neat side effects and high-quality etching results. The selection of nozzle structure and spraying method must be rigorous.
11. Many theories and design methods have emerged to achieve favorable side effects. Despite variations, all etching theories acknowledge a basic principle: maintaining metal surface contact with fresh etching solution rapidly. Chemical analysis supports this, indicating ammonia’s (NH3) role in determining etch rates.
12. Traditionally, lower monovalent copper ion content in ammonia etching solutions correlates with faster reaction speeds, validated through industry experience. Some ammonia-based products incorporate specialized ligands to reduce monovalent copper ions, enhancing reactivity.
13. The presence of monovalent copper ions, tightly bound within ammonia’s complexing groups, poses challenges. Introducing air into the etching chamber facilitates their removal by converting them into divalent copper. However, excessive air can accelerate ammonia loss and reduce pH, thereby decreasing etch rates.
14. In chemical etching (PCH), which is advancing into machine structure design, solutions use divalent copper instead of ammonia-copper etching. This method, applicable to the printed circuit industry, imposes stringent etching requirements, especially regarding copper foil thickness (typically 5 to 10 mils).
—
These revisions aim to enhance clarity and readability while maintaining the technical accuracy and flow of the original text.
Currently, the predominant method in PCB fabrication is the “pattern plating method.” This approach begins by applying a layer of lead-tin corrosion-resistant material onto specific areas of the copper foil intended to remain on the outer layer, forming the circuit pattern. Subsequently, the remaining copper foil is chemically dissolved away in a process known as etching.
Types of Etching
It is important to note that during the etching process, the board typically features two layers of copper. In outer layer etching, only one layer of copper is selectively dissolved, leaving behind the required circuit pattern. This pattern plating method ensures that copper plating exists solely beneath the lead-tin resist layer.
Alternatively, another method involves plating copper across the entire board, with areas not covered by photosensitive film treated only with tin or lead-tin resist. Known as the “full board copper plating process,” this method requires copper plating over the entire board surface, followed by complete etching of unwanted copper. Unlike pattern plating, this approach demands two rounds of copper plating across the board, increasing complexity. It becomes particularly problematic with fine wire widths, leading to issues such as side corrosion that can significantly impact line uniformity.

1. In the processing technology of the outer circuit of the printed circuit board, another method involves using photosensitive film as the resist layer instead of metal coating. This method closely resembles the inner layer etching process and can be referenced during inner layer manufacturing.
2. Currently, tin or lead-tin is the most commonly used corrosion-resistant layer in the etching process involving ammonia-based etchants. Ammonia-based etchants are widely used chemical solutions that do not undergo chemical reactions with tin or lead-tin. Ammonia etchants primarily consist of ammonia/ammonium chloride etching solutions.
3. Additionally, ammonia/ammonium sulfate etching chemicals are available in the market. Copper can be separated from sulfate-based etching solutions through electrolysis, allowing for reuse. Due to its low corrosion rate, it is less common in actual production but is anticipated for chlorine-free etching.
4. Some have attempted to use sulfuric acid-hydrogen peroxide as an etchant for corroding outer layer patterns. However, due to reasons such as economic viability and the management of waste liquids, this process has not gained widespread commercial use. Moreover, sulfuric acid-hydrogen peroxide cannot be used for etching lead-tin resist, making it a secondary method in outer layer PCB production, thus attracting minimal attention.
5. Etching Quality and Previous Issues
6. The fundamental requirement for etching quality is the complete removal of all copper layers except those under the resist layer. Strictly speaking, etching quality encompasses wire width consistency and the degree of undercutting. Given the characteristics of current etching solutions, side etching in the horizontal directions is nearly unavoidable.
7. Undercutting is a critical etching parameter often discussed, defined as the ratio of undercut width to etch depth, known as the etching factor. In the printed circuit industry, this factor varies widely, from 1:1 to 1:5. A minimal undercut or low etching factor is generally preferred.
8. Equipment Adjustment and Interaction with Etching Solutions
9. In printed circuit processing, ammonia etching involves delicate and complex chemical reactions. Once optimized, production can proceed smoothly. Continuous operation is crucial; halting and drying are not advisable. The effectiveness of the etching process largely depends on equipment performance.
10. Regardless of the etching solution used, high-pressure spraying is essential for achieving neat side effects and high-quality etching results. The selection of nozzle structure and spraying method must be rigorous.
11. Many theories and design methods have emerged to achieve favorable side effects. Despite variations, all etching theories acknowledge a basic principle: maintaining metal surface contact with fresh etching solution rapidly. Chemical analysis supports this, indicating ammonia’s (NH3) role in determining etch rates.
12. Traditionally, lower monovalent copper ion content in ammonia etching solutions correlates with faster reaction speeds, validated through industry experience. Some ammonia-based products incorporate specialized ligands to reduce monovalent copper ions, enhancing reactivity.
13. The presence of monovalent copper ions, tightly bound within ammonia’s complexing groups, poses challenges. Introducing air into the etching chamber facilitates their removal by converting them into divalent copper. However, excessive air can accelerate ammonia loss and reduce pH, thereby decreasing etch rates.
14. In chemical etching (PCH), which is advancing into machine structure design, solutions use divalent copper instead of ammonia-copper etching. This method, applicable to the printed circuit industry, imposes stringent etching requirements, especially regarding copper foil thickness (typically 5 to 10 mils).
—
These revisions aim to enhance clarity and readability while maintaining the technical accuracy and flow of the original text.